The Ministry of Health and Prevention, MoHAP received reports issued by the World Health Organization (WHO) indicating the presence of the N-Nitrosodimethylamine (NDMA) impurity at higher levels than the permitted rates in some products of the manufacturing companies for Metformin. The drug is prescribed to treat patients with diabetes type 2.
MoHAP underlined that all the precautionary measures have been taken for patient’s health and safety, pointing out that it constantly follows up on the proportions of the raw material of NDMA which is imported by the manufacturing companies of Metformin drug. MoHAP added that it also follows up with the drug agents and their companies to confirm NDMA proportions in Metformin preparations which are imported to the country as an end product.
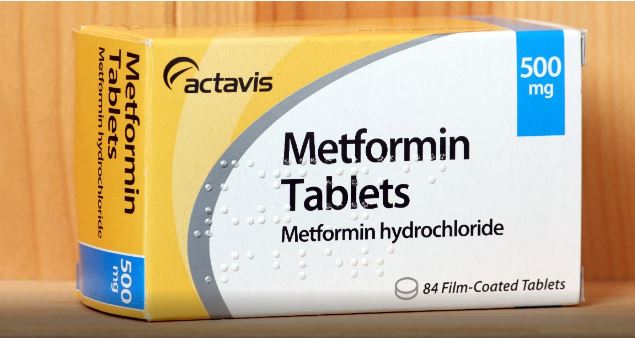
NDMA found at low levels in numerous items of food and some drinking-water, as well as polluted air. The exposure to this substance at higher levels more than acceptable daily intake and for very long periods can increase cancer risk according to the International Agency for Research on Cancer (IARC).
Analysis should be carried out in any qualified laboratory while ensuring that the recommended method of analysis is followed by the FDA, according to the minimum acceptable dose per day – manufacturing companies should submit the required reports within six months of the date of this circular, the end of the current month as a deadline.
The Health Ministry stressed that it has not yet been found that there are unacceptable proportions in the Metformin products in the country and that it still ask companies to provide the ministry with the results of the approved analyses to ensure that there is no NDMA impurity in the drugs imported to the country.
The Health Ministry urged community members and health practitioners to immediately report any side effects of drugs or poor-quality medicines through the UAE RADAR portal at http://www.mohap.gov.ae/ar/services/Pages/406.aspx
MoHAP conducts all required analysis
Dr. Amin Hussein Al Amiri, the Assistant Undersecretary of MoHAP’s Public Health Policy and Licenses, confirmed that MoHAP is still conducting all the required analyses for the pharmaceutical preparations that contain NDMA substance and that the ministry will instantly announce the findings of these analyses on those products containing NDMA impurity which will be withdrawn from the market.
Al Amiri urged patients not to stop taking Metformin drug or adjusting dose without consulting their attending physicians.
He also recommended the health practitioners not to stop prescribing Metformin drug until they get any updated information from MoHAP.
Communication with world drug organizations
Al Amiri also said that MoHAP communicates on daily basis with the Metformin-related international organizations, such as the US Food and Drug Administration (FDA), European Medicines Agency, and the Therapeutic Goods Administration (TGA) in Australia to investigate warnings related to any pharmaceuticals.
This comes as part of MoHAP’s keenness on enhancing patient’s health and safety and building quality and safety for therapeutic, healthcare, and pharmaceutical systems according to international standards.




![The Top & Most Popular Seafood Bucket Restaurants in Dubai for you [Never Miss]](https://uae24x7.com/wp-content/uploads/2020/09/8-seafood-in-a-bucket-scaled-e1600739237403.jpg)
![Procedures for Renewing the Driving License in Abu Dhabi [3 Simple Steps]](https://uae24x7.com/wp-content/uploads/2020/07/Capture-9-e1595666454466.jpg)





